Breast cancer screening: thermogram no substitute for mammogram
U.S. Food and Drug Administration (FDA)
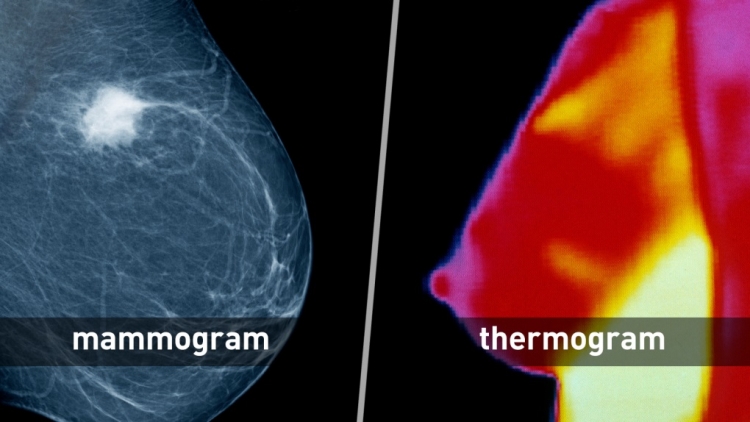
Proper breast cancer screening checks for cancer even before there may be signs and symptoms of the disease. Mammography (low-dose X-ray imaging of the breast) is still the most effective primary screening method for detecting breast cancer in its early, most treatable stages.
Some health centers provide information that can mislead patients into believing thermography, a type of test that shows patterns of heat on or near the surface of the body, is a proven alternative to mammography. But the U.S. Food and Drug Administration is not aware of any scientific evidence to support these claims.
Thermography has not been shown to be effective as a standalone test for breast cancer screening and detecting early-stage breast cancer.
Mammography vs. Thermography
The FDA regulates the medical devices used for breast cancer screening.
About 1 in 8 women in the U.S. will be diagnosed with breast cancer sometime in their lives, reports the National Cancer Institute. Men can develop breast cancer, but it happens much less often than in women. Early detection of breast cancer by using mammography reduces the risk of breast cancer death and increases treatment options, according to the American Cancer Society. One of the greatest risks from thermography is that those who opt for this method instead of mammography may miss the chance to detect breast cancer at its earliest stage.
Thermography devices have only been cleared by the FDA as an “adjunctive” tool, referring to use alongside a primary screening test like mammography. Patients who undergo a thermography test alone should not be reassured of the findings because the device was not cleared to be used without another testing method like mammography.
Moreover, some websites claim thermography can find breast cancer years before it would be detected through other methods, and they have unproven claims about improved detection of cancer in dense breasts using thermography. The FDA is not aware of any evidence that supports these claims.
The FDA has taken, and will continue to take, appropriate regulatory action against thermography device manufacturers who market thermography devices as an alternative to mammography.
Advice for Patients Getting Breast Cancer Screening
Some women have sought out thermography because it is painless and does not require exposure to radiation.
If you are worried about how a mammogram feels, talk to your health care professional about what you can expect. A mammogram can be uncomfortable for the person being screened because it briefly presses on the breast to spread the breast tissue and increase the clarity of the X-ray image.
Also talk to your health care professional if you have specific questions about mammography, including questions about when and how frequently you should be screened. As a rule, also call your health care professional if you notice any change in either of your breasts such as a lump, area of thickening of or leakage from the nipple, or changes in how the nipple looks.